Articles from Siemens Healthineers

Siemens Healthineers announced today its Innovance Antithrombin assay has achieved FDA clearance for a new claim allowing it to be used as a companion diagnostic test for people receiving treatment with Qfitlia™ (fitusiran), a Sanofi hemophilia therapy.1 Hemophilia is a lifelong genetic bleeding disorder that significantly affects the day-to-day lives of people living with the disease, creating potentially life-threatening risks from otherwise normal situations experienced by those without hemophilia. The body’s inability to clot blood effectively can prolong bleeding after injuries, result in excessive bruising and joint pain, and increase risks during surgery or other medical procedures.
By Siemens Healthineers · Via Business Wire · March 28, 2025

New research conducted by YouGov on behalf of Siemens Healthineers1 reveals Americans’ devotion to football fandom could put their heart health at risk. According to the survey, roughly 1 in 5 Americans (21%) would hesitate to leave a professional sporting event to go to the hospital if they suspected they were having a heart attack. Among American football fans, however, 28% would hesitate to leave a professional sporting event to go to the hospital if they suspected they were having a heart attack. This number only declined to 19% among football fans who themselves have already visited the ER with symptoms of a heart attack.
By Siemens Healthineers · Via Business Wire · January 23, 2025

Siemens Healthineers now offers Atellica Integrated Automation on the Atellica CI Analyzer,1 to consolidate 25 tasks typically handled manually by clinical laboratory staff. This offering redefines automation for clinical labs and has been shown to reduce the manual workflow steps laboratory professionals perform by 75%.2
By Siemens Healthineers · Via Business Wire · October 30, 2024

A new prognosis claim for the Siemens Healthineers Atellica IM High-Sensitivity Troponin I (TnIH) test1 immediately advances care for at-risk cardiac patients in the U.S. The easy blood test helps healthcare providers identify patients at risk of death and major cardiac events that could occur up to one year after presenting to the emergency department with signs and symptoms of acute coronary syndrome. It is the first test of its kind in the United States to receive FDA clearance for prognosis.
By Siemens Healthineers · Via Business Wire · October 15, 2024
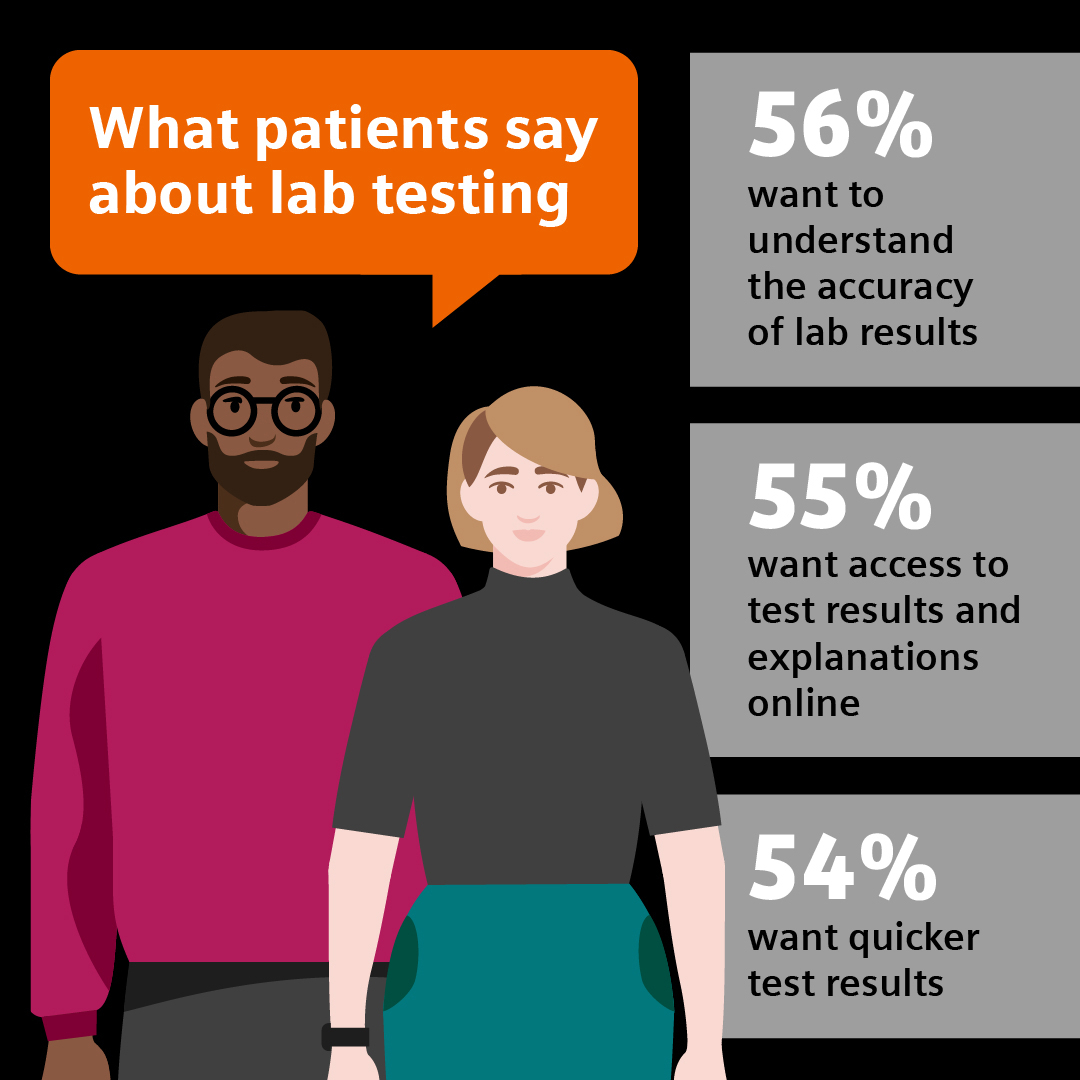
A new survey from Siemens Healthineers and The Harris Poll1 reveals the toll prolonged understaffing is taking on clinical laboratory personnel and the patients they serve. As reliance on laboratory diagnostic testing in the U.S. healthcare system continues to grow, so does the pressure on laboratory professionals who process more than 14 billion tests ordered annually.2 These essential workers are dedicated to a high-quality standard for patients. New data reveals how burnout can hinder their ability to maintain this standard. Automation is widely believed to be a key element in solutions to address some of the major challenges.
By Siemens Healthineers · Via Business Wire · July 30, 2024

ADLM 2024 Clinical Lab Expo--Siemens Healthineers has pushed the boundaries of medical engineering to improve patient care for more than 125 years. At the ADLM 2024 Clinical Lab Expo (from July 30-Aug. 1 at McCormick Place, Chicago, Booth #501), Siemens Healthineers will demonstrate what’s achievable for laboratory testing with human-centered engineering and automation. New capabilities from Siemens Healthineers bring unmatched automation to laboratories of all sizes. Enhancements to the Atellica Portfolio underscore sustainability, leading workflow efficiency, AI-enabled intelligence, and advanced analytics to turn data into practical clinical insights.
By Siemens Healthineers · Via Business Wire · July 24, 2024

Siemens Healthineers and UHealth - University of Miami Health System announced a Value Partnership1 agreement. This strategic relationship will further technological advancement and standardization of equipment at the health system, while allowing the University to create educational and training programs for clinicians and technologists. This unique agreement is the first of its kind in Florida.
By Siemens Healthineers · Via Business Wire · October 31, 2022

Siemens Healthineers and The Ohio State University Wexner Medical Center announced a new alliance, aimed at advancing personalized medicine and improving access to high quality, cost-effective healthcare. Through the five-year strategic partnership, Siemens Healthineers and Varian, a Siemens Healthineers company, will provide comprehensive technology and services that will build on previous successful collaborative projects. The university and medical center will contribute research initiatives from scientists, physicians and patients. This work forms a living lab, a place where early scientific validation will speed breakthroughs in individualized medicine and health care delivery.
By Siemens Healthineers · Via Business Wire · June 28, 2022
Siemens Healthineers announces that the U.S. Food and Drug Administration (FDA) today granted Emergency Use Authorization (EUA) for the CLINITEST Rapid COVID-19 Antigen Self-Test,1, 2, 3 providing nationwide access to a new at-home or over-the-counter self-test as COVID-19 testing needs continue to grow for individuals, families, and businesses. The easy-to-use nasal swab test is intended to aid in the rapid detection of SARS-CoV-2 (the virus that causes COVID-19) and provides visually read test results in just 15 minutes. It is authorized for self-testing use by individuals age 14 and older or adult-collected samples from individuals ages 2 to 13 years. The test is expected to be available starting in January. Siemens Healthineers has secured dedicated production capacity for U.S. bound product in the tens of millions per month.
By Siemens Healthineers · Via Business Wire · December 29, 2021

Siemens Healthineers is proud to be recognized in Stage I of the Foundation for the National Institutes of Health (FNIH) Biomarkers Consortium Non-Invasive Biomarkers of Metabolic Liver Disease (NIMBLE) study. The data from “LO1: Primary Results of the NIMBLE Stage 1-NASH CRN Study of Circulating Biomarkers for Nonalcoholic Steatohepatitis and its Activity and Fibrosis Stage,” presented in a late-breaking oral presentation at The Liver Meeting 2021 on Sunday, November 14, show that Siemens Healthineer’s ELF™ Test performed the best of the five blood biomarkers selected for the study. The ELF Test was one of three biomarkers in the study that met the criteria for the successful identification of fibrosis stage ≥2. The performance of the ELF Test further improved for fibrosis stage ≥3 and stage 4.
By Siemens Healthineers · Via Business Wire · November 15, 2021

Siemens Healthineers announced today that its Enhanced Liver Fibrosis (ELF™) Test was granted marketing authorization under the De Novo review pathway.1 The ELF Test, for use with the ADVIA Centaur® XP Immunoassay System, provides a simple numeric score that is automatically generated via an algorithm and is used to improve patient care by assessing the likelihood of progression to cirrhosis and liver-related clinical events in patients with advanced fibrosis (F3 or F4) due to non-alcoholic steatohepatitis (NASH).
By Siemens Healthineers · Via Business Wire · August 24, 2021

Siemens Healthineers announces the Food and Drug Administration (FDA) clearance of the MAGNETOM Free.Max, a new High-V magnetic resonance (MR) scanner that combines a 0.55 Tesla (0.55T) field strength with deep learning technologies and advanced image processing. By doing so, the scanner broadens the range of MR clinical applications and provides customers with the inherent clinical benefits of a mid-field MR scanner. The first and only 80 cm wide-bore system available, the MAGNETOM Free.Max also facilitates MR scanning for extremely obese and claustrophobic patients, enhancing the patient experience.
By Siemens Healthineers · Via Business Wire · July 6, 2021
